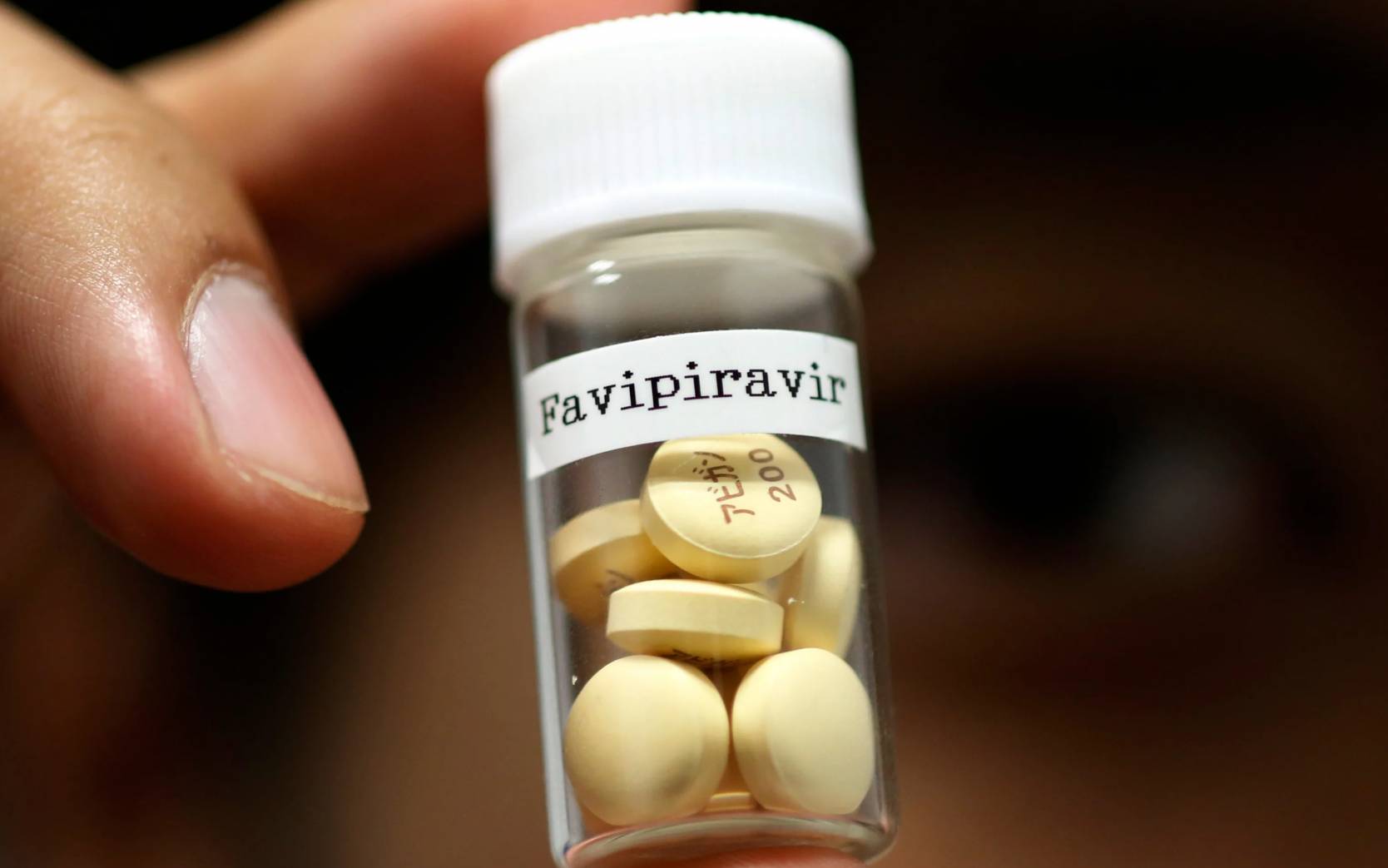
The Ministry of Health is in talks with the Japanese government to try an antiviral drug, used against influenza, in the treatment of the coronavirus.
Though not among the four drugs and drug combinations commissioned by the World Health Organisation (WHO), under the Solidarity Trials 1, favipiravir is said to have shown promising results when used in patients with the coronavirus.
The drug, under the trade name Avigan, is said to affect an enzyme that helps the virus to replicate and multiply inside the human cell. It is now seen as a potential treatment for “neglected and emerging RNA viruses” like the coronavirus.
Promising results
However, there is limited evidence and data on the use of the drug in coronavirus patients, including when to administer it and to whom.
The news was revealed yesterday by Health ministry Director-General Patrick Amoth, who said discussions between the two countries were underway.
The drug has shown some promising results on Covid-19 cases in Japan and China.
The drug is developed by the Japanese pharmaceutical Toyama Chemicals of Fujifilm Corporation.
Dr Amoth said the ministry is seeking to have limited doses of the drug, which will be an extension of the ongoing clinical trials.
“We are hopeful we will also get stellar results like what they got in Japan and China,” said Amoth.
The drug has been proven to treat Covid-19 patients in four days compared to 11 days when not treated with the drug.
Lung function
The drug improves lung function in about nine out of ten patients who use it. Fujifilm Corporation will be kicking off a phase II trial of the drug in the United States as phase III is underway in Japan.
While the drug was developed and registered as flu medicine, it has to undergo several clinical trials and another approval in order to be registered as a remedy for Covid-19.
So far, there is no registered drug or vaccine for the disease but clinical trials are also underway for hydrochloroquine as a potential treatment.
Japanese media reported on Sunday that Japan plans to store 2 million doses of Avigan, an increase from its current level of 700,000.
Tests in China have found that the medication is useful in treating Covid-19 in its early stages and preventing the disease from worsening.
Malaria drug
According to i24 News, Israel is among the first countries in the world to receive the experimental Japanese drug to treat the coronavirus, which will be tested in hospitals across the country.
While announcing trials of the four drugs, under the Solidarity Trials 1, WHO Director-General Tedros Adhanom, said in a press briefing “each new patient who joins the trial gets us one step closer to knowing which drugs work.”
Hydrochloroquine, which is an old malaria drug has already received an Emergency Authorisation Use from Foods and Drug Administration (FDA) in the United States for Covid-19 cases, a push engineered by US President Donald Trump.
Sarilumab, or known as Kevzara, also has several claims behind it as an antibody-drug, which can treat the disease. Studies are underway at Tuft Medical Centre even though the drug is approved for arthritis.
Another drug, Remdesivir, developed for Ebola virus is also being studied for its efficacy against Covid-19 after showing some promises on animals infected with SARS and MERS in the US and also Israel.
The World Health Organisation recognises that there is no vaccine or registered treatment for the coronavirus yet.
It will take up to 18 months, according to FDA, for a vaccine to be approved and mass production is kick-started.
WHO has guidelines in place for countries which are developing treatment models and vaccines, which it says, will be updated regularly.
“WHO is working with its networks of researchers and other experts to coordinate global work on surveillance, epidemiology, mathematical modelling, diagnostics and virology, clinical care and treatment, infection prevention and control, and risk communication," says WHO report.
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.