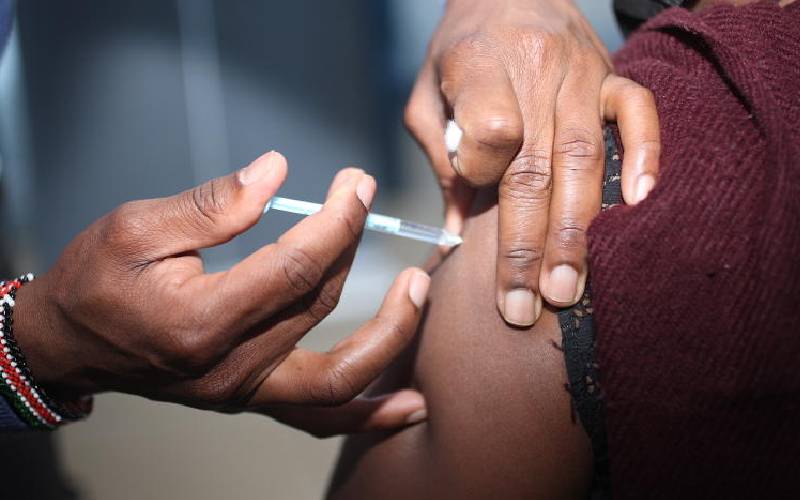
Some Kenyans fancy getting the AstraZeneca jab then Pfizer “to boost immunity” but mixing and matching of vaccine doses is discouraged by both the Ministry of Health and the World Health Organisation (WHO).
So far Kenya has received four vaccines; AstraZeneca, Moderna, Johnson & Johnson and Pfizer. Sinopharm from China is also expected, and with these options, Kenyans are still wondering why they cannot mix them.
Facts First
This story continues on The Standard INSiDER. Subscribe now for unfiltered journalism that holds power to account.
Already have an account? Login
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.











