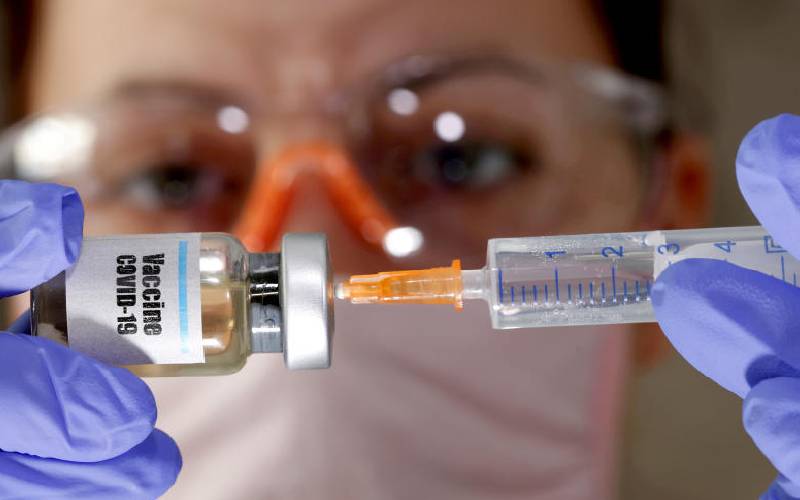
People in developing countries should receive news of a breakthrough in the search for a Covid-19 vaccine with cautious optimism if the number of doses projected versus the demand is anything to go by.
In what was hailed as a ‘great day for science and humanity’, news of the vaccine that offers over 90 per cent protection yesterday breathed life into the race towards eradication of the pandemic through mass vaccination globally.
Facts First
This story continues on The Standard INSiDER. Subscribe now for unfiltered journalism that holds power to account.
Already have an account? Login
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.











