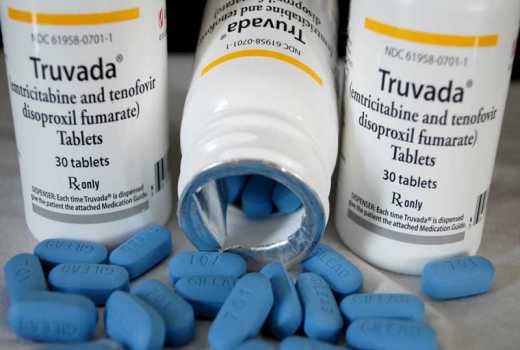
Kenya is among three African countries that will participate in a radical makeover of the slow-moving daily HIV prevention pill Truvada.
Users of the pill, officially launched in Kenya last year say its colour, size, packaging and daily frequency are all great put offs.
In response, USAid has provided about Sh1.19 billion ($10 million) for the development of a discreet drug delivery technology to be tried in Kenya, South Africa and Uganda.
The project will develop a drug delivery patch than can be placed discreetly on the skin programmed to deliver a specific dose of Truvada into the blood stream.
The Americans think the technology called microarray patch will overcome some of the problems that have discouraged the uptake of the pill.
HIV experts say some Truvada users complain about the colour, size and even the rattling sound that the tablet makes when being taken out of its plastic container.
Dr Nelly Mugo of the Kenya Medical Research Institute recently told journalists that some users complain that people mistake them of taking HIV treatment medication.
The pill, Dr Mugo, a strong advocate of Truvada, said has also been linked to the sex stimulant Viagra and another drug used for mental health because of their blue colour.
"We have had several meetings with the manufacturers on these concerns,” Dr Christine Ogolla, a programme director at the Elizabeth Glaser Aids Foundation told journalists in Nairobi recently.
A statement released last week by Queen’s University Belfast, Northern Ireland, the lead agency in the new project says they will work mainly with women in the three countries to address the concerns.
“The patches will be a discreet, easy-to-use technology that contains tiny projections that painlessly penetrate the top layer of skin to deliver the drug,” says the university.
Poor adherence
The patch, if it works, will overcome the problem of colour, size and rattling while releasing the drug over several weeks or months instead of the current practice of swallowing the pill daily which is a cause for poor adherence.
Others in the project are the pharmaceutical companies ViiV Healthcare and LTS Lohmann Therapie-Systeme AG and the NGOs PATH and Population Council.
So far data shows only about 13,000 Kenyans have taken up the pill against a Ministry of Health five-year target of 500,000 users.
The main driver of Truvada, the US President’s Emergency Plan for Aids Relief, targets at recruiting about 5,000 users in Kenya in 2018 and about 40,000 in the next few years.
Truvada is manufactured by the American pharmaceutical company Gilead Sciences, which reported global sale revenues in excess of $10.2 billion from the drug between 2014 and 2016.
The 2016 total sale revenue for Gilead, from all products, is reported at $30.39 billion or slightly more than Sh3 trillion.
Health experts are also grappling with the requirement that Truvada users be tested for kidney function at least three times annually which they say could be expensive and burdensome for local health systems.
A study published earlier this month by Mugo, among others, suggests that the frequency of kidney testing could be safely reduced to twice annually.
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.