×
The Standard e-Paper
Stay Informed, Even Offline
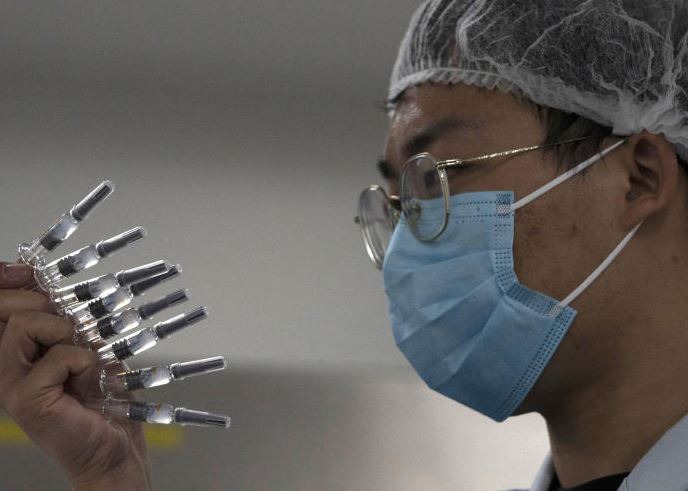
Provincial governments across China are placing orders for experimental, domestically made coronavirus vaccines, though health officials have yet to say how well they work or how they may reach the country’s 1.4 billion people.
Developers are speeding up final testing, the Chinese foreign minister said during a UN meeting last week, as Britain approved emergency use of Pfizer Inc.’s vaccine candidate and providers scrambled to set up distribution.