×
The Standard e-Paper
Home To Bold Columnists
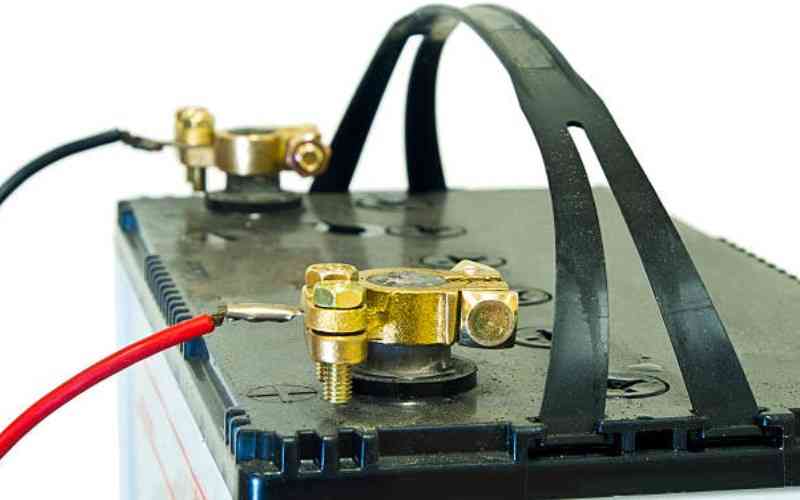
In the ever-evolving world of energy storage the humble lead-acid battery has remained a stalwart withstanding the tests of time.
Inexpensive and dependable, lead acid batteries have been around for more than 100 years, including being used in the early versions of electric vehicles back in the 1890s.