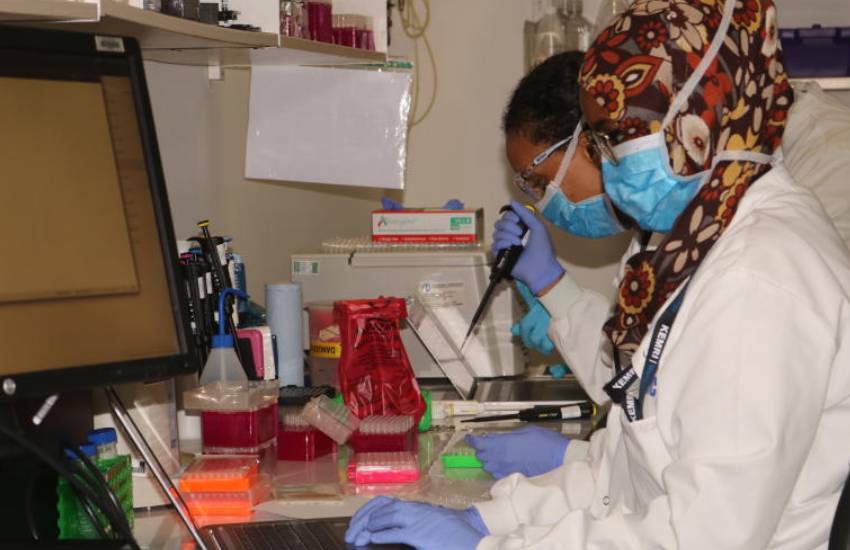
Pharmaceutical companies in Kenya import about 80 per cent of the medicine used here. Most comprise generics and originals. For a drug to be safe for human use it has to go through clinical trials, testing and approvals from various bodies. However, majority of the clinical trials are conducted in foreign countries with very few Africans.
Consider the drug trials that took part in 2019 before the corona pandemic: 48 drugs were put for trial and 46,391 patients from various ethnic groups participated. Of the number, 72 per cent were whites and 18 per cent Hispanics. Only nine per cent were of Black African American descent, the same percentage as Asians. Up to 72 per cent were women.