×
The Standard e-Paper
Join Thousands Daily
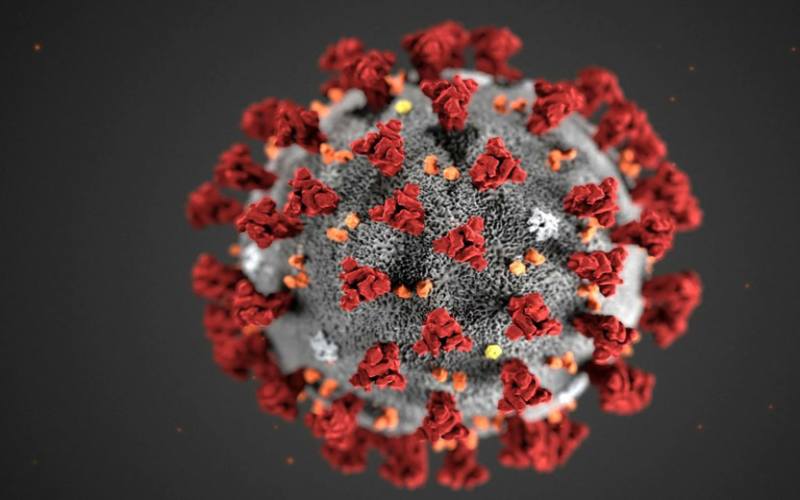
Monoclonal antibodies bind to certain proteins on a virus, neutralizing its ability to infect human cells. [AFP]
The United States is working with a pharmaceutical company to develop a treatment for the 2019 Novel Coronavirus, using a class of drug that has boosted survival rates among Ebola patients, officials said Tuesday.